DISCOVER WHAT NUTRACEUTICALS IS
A biotech company focused on developing natural solutions.
We are preparing an IND for an inhaled biologic targeted at acute anxiety, delivered via our pMDI platform in collaboration with university partners. The program moves from pre-clinical to clinical phases with an expected IND completion target of November 2025.
Program Overview
Study Goals
This study aims to evaluate the effectiveness of an inhaled drug called NC-107 in reducing the severity of anxiety symptoms in adults with Generalized Anxiety Disorder (GAD). It also aims to assess the safety and pharmacokinetics (absorption and distribution in the body) of NC-107.
Benefits
Participants will receive close monitoring of their anxiety symptoms and overall health throughout the study. They may experience a reduction in their anxiety symptoms if assigned to the NC-107 group. Their participation will contribute to the development of a potential new treatment option for GAD.
Study Design
Participants aged 18 or older with moderate GAD will be enrolled and randomly assigned to receive either NC-107 or a placebo (inactive substance) inhaler for 4 weeks. Participants will take two puffs from the inhaler twice daily. Anxiety symptom assessments, vital signs, and blood tests will be conducted at regular intervals throughout the study. Blood samples will be taken to measure the levels of NC-107 in the body.
Risks
Potential risks associated with participation include side effects from the investigational drug NC-107, which are currently unknown. There may also be risks associated with blood draws, such as bruising or infection at the puncture site.
Understanding our Devices
Problem Statement:
Despite decades of use and widespread adoption, pressurized metered-dose inhalers (pMDIs) remain largely unchanged in form and function—relying on aging delivery systems, environmentally harmful propellants, and inconsistent patient adherence due to usability challenges. As the global burden of respiratory diseases such as asthma and COPD continues to rise, especially among pediatric and elderly populations, current pMDIs fall short in addressing key issues such as:
•Environmental impact of hydrofluoroalkane (HFA) propellants.
•Lack of real-time dose tracking and feedback, leading to misuse and non-adherence.
•Limited child safety features, despite high usage among pediatric patients.
•Insufficient innovation in ergonomics and intuitive design, particularly for first-time users and vulnerable populations.
This growing disconnect between clinical demand, patient needs, and available technology highlights an urgent opportunity—and responsibility—for stakeholders to reimagine the pMDI: making it smarter, safer, and more sustainable for the next generation of respiratory care.
The pMDI (pressurized metered-dose inhaler) market is witnessing steady growth, driven primarily by the increasing prevalence of respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD), as well as ongoing advancements in inhaler technology.
Market Size and Growth:
Market Size:
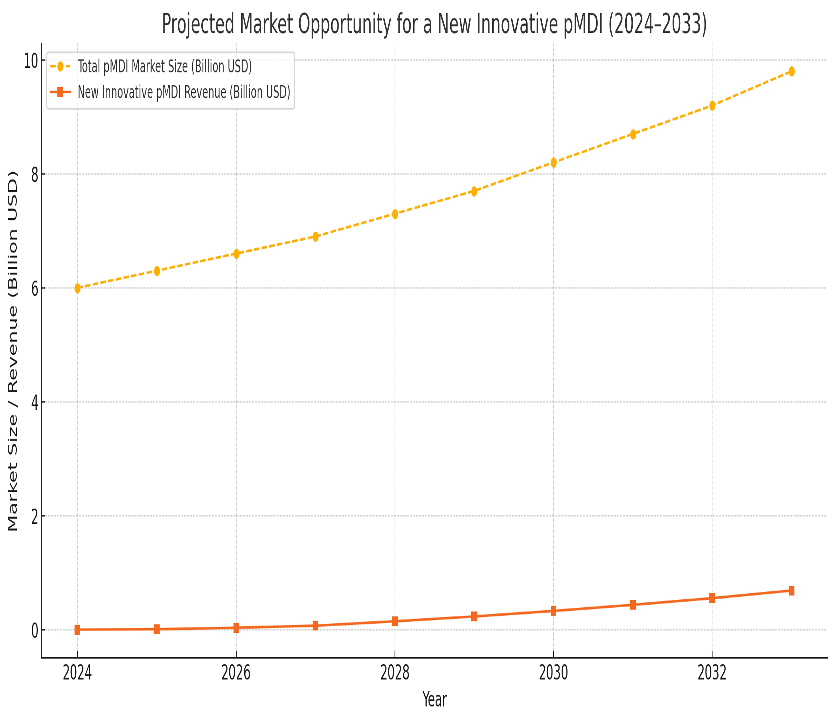
1.The global pMDI market was valued at approximately USD 6.03 billion in 2023.
2.Another projection places the 2024 market at USD 5.96 billion, with growth to USD 9.21 billion by 2033, corresponding to a CAGR of 5.6% between 2025 and 2033.
Growth Drivers:
1.The rising burden of respiratory diseases worldwide is one of the key factors driving market growth. The number of asthma and COPD cases is increasing, especially in aging populations, which increases the demand for pMDIs.
2.Technological advancements in pMDIs, including dose counters, new propellants (such as HFA alternatives to CFCs), and user-friendly designs, are making these inhalers more effective and appealing to both healthcare providers and patients.
3.Environmental regulations, such as the phasing out of CFCs in favor of HFA propellants, have spurred innovation in device technology and alternative delivery methods.
Regional Breakdown:
1.North America holds the largest market share for pMDIs, driven by a high prevalence of respiratory diseases and established healthcare infrastructure. The market in North America is projected to grow at a CAGR of 4.0% from 2024 to 2031.
2.Europe and Asia-Pacific are also significant regions, with increasing adoption of inhalation therapies and rising awareness of respiratory health.
Opportunity for New Innovative pMDIs:
1.With increasing market size, there is significant opportunity for innovative products that address specific patient needs. For example, devices that offer improved ease of use, personalized dosage, and environmentally friendly propellants are likely to gain traction.
2.New entrants in the pMDI market can capture increasing market share by addressing gaps in current offerings, such as affordability, ease of use for pediatric and elderly populations, and new formulations that improve patient adherence.
Conclusion:
The pMDI market presents a growing opportunity for new, innovative devices and formulations. The demand for these products will continue to rise as healthcare systems focus on managing respiratory diseases more effectively. The growing market size, paired with increasing adoption and technological advancements, makes this a promising area for investment and product development.